Hydroxyl Radical Formation on Metal-Loaded Ga2O3 Photocatalysts for Dehydrogenative Coupling of Methane to Ethane with Water
F. Amano, M. Ishimaru
Energy Fuels 2022, 36, 10, 5393-5402, https://doi.org/10.1021/acs.energyfuels.2c00401
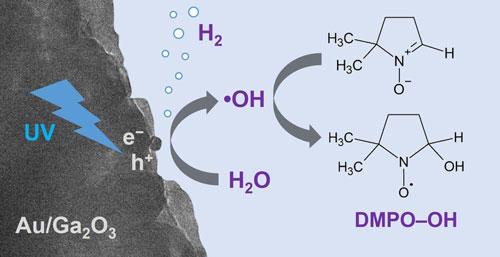
Photocatalytic non-oxidative coupling of methane (photo-NOCM; 2CH_{4} → C_{2}H_{6} + H_{2}) can directly convert methane into ethane and hydrogen at room temperature. However, the apparent quantum efficiency (AQE) of photo-NOCM is very low in the absence of oxidants. We observed that photocatalytic dehydrogenative coupling of methane (photo-DHCM) proceeds in the presence of water vapor using Ga_{2}O_{3}-based photocatalysts under ultraviolet (UV) light irradiation. Photo-DHCM is efficiently induced over Pt/Ga_{2}O_{3} and Pd/Ga_{2}O_{3} photocatalysts, accompanied by steam reforming of methane (photo-SRM). For C_{2}H_{6} production, the AQE of photo-DHCM with water vapor was more than 2 orders of magnitude higher than that of conventional photo-NOCM. However, the role of the metal co-catalyst supported on Ga_{2}O_{3} for the production of C_{2}H_{6} and H_{2} in photo-DHCM is unclear. The reaction mechanism accelerated by water vapor was assumed in which CH_{4} was activated by a hydroxyl radical (~{•}OH) and homocoupling occurred by the two methyl radicals. Therefore, ~{•}OH formation affects the productivity and selectivity of the photocatalytic CH_{4} conversion. In this study, we investigated the effect of metal co-catalysts (Au, Ag, Ru, Rh, Pd,and Pt) supported on a Ga_{2}O_{3} photocatalyst on ~{•}OH formation by an electron spin resonance (ESR) method using a spin-trapping agent. ESR measurements upon UV irradiation revealed that the ~{•}OH concentration was high in Au/Ga_{2}O_{3}, which exhibited a high C_{2}H_{6} production rate (1140 μmol gcat-1 h-1; AQE = 4.3% at 254 nm) and high C_{2}H_{6} selectivity (92.2% on a carbon basis). In contrast, Rh/Ga_{2}O_{3}, which had the lowest ~{•}OH concentration, exhibited high selectivity for photo-SRM and water-splitting reactions compared to photo-DHCM.